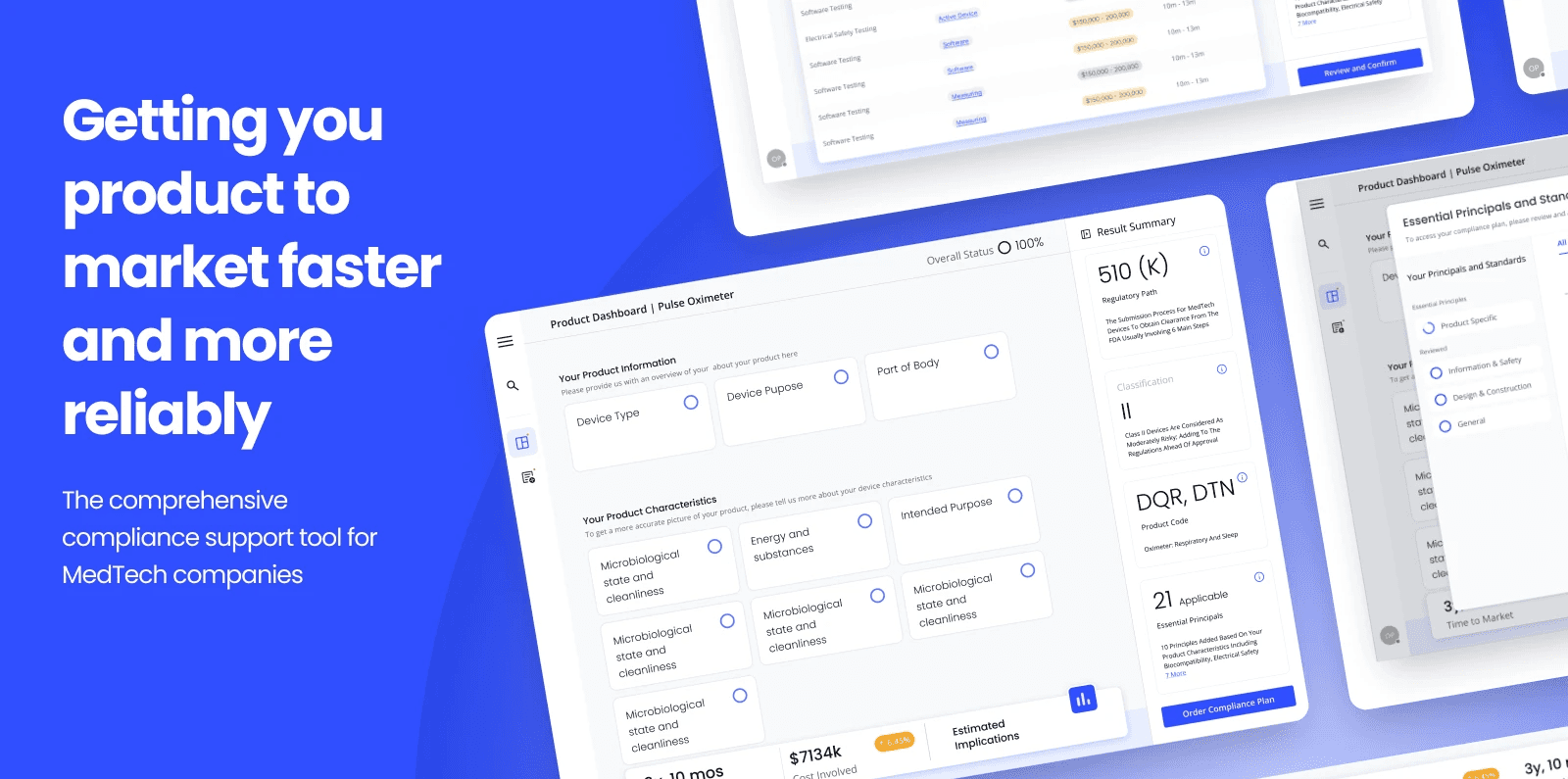
Compliance support tool for MedTech companies
Case Study: Compliance support tool for MedTech companies
(Confidential and sanatised)
Introduction: In the ever-evolving landscape of medical technology, compliance with regulatory standards is paramount. Our client - GlobalTech solutions company, embarked on a mission to develop a digital product aimed at streamlining regulatory processes, thereby accelerating product launches while ensuring adherence to regulations. Focused initially on the medical technology sector, our goal was to provide a comprehensive platform for test and compliance services, facilitating efficient planning and execution throughout the product lifecycle.
Challenges Faced: The complexity of regulatory requirements poses significant challenges for companies in the med tech industry. Understanding what needs to be tested and how to conduct these tests efficiently proved to be particularly daunting. Additionally, integrating regulatory compliance seamlessly into the product development process presented its own set of hurdles.
Objectives: Our primary objective was to create a digital platform that serves as a centralised hub for managing test plans, tracking status, and recording results.
We aimed to define a long-term compliance strategy from the early stages of research and development, with automation capabilities for re-certification as regulations evolve. Furthermore, we sought to offer our expertise and data to assist clients in selecting materials and suppliers that align with their R&D objectives.
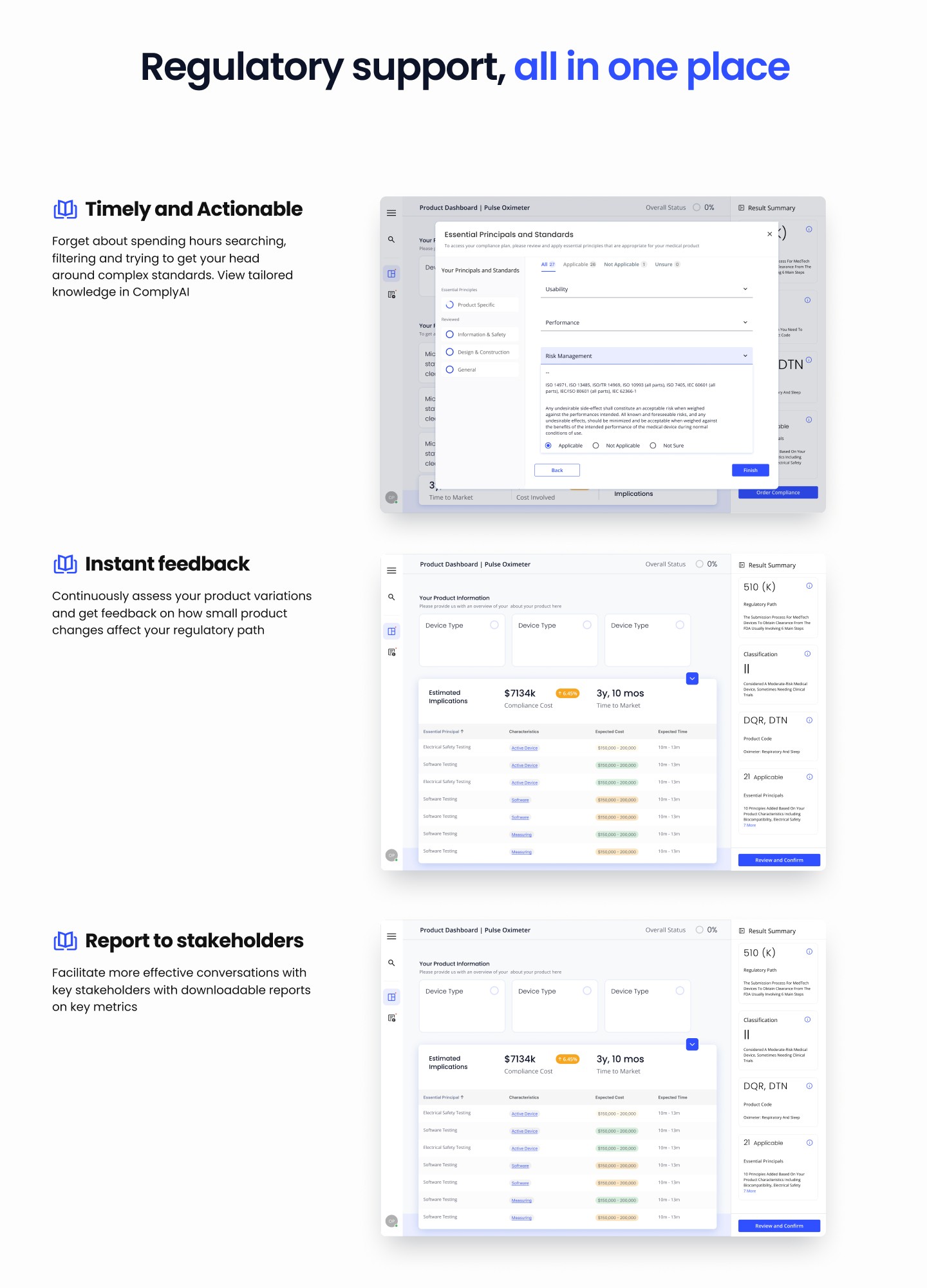
My Role and Methodology: As a UI/UX designer, my role in Phase 2 of the project was multifaceted, encompassing various aspects crucial to the success of the digital product development:
User Interviews with Strategic Planning: I meticulously planned and executed user interviews, employing strategic methodologies to elicit insightful feedback from participants. I used quick prototypes links for user to go through them and shadowing there observations and requirements.
User-Centric Design Principles: Employing a user-centric approach, I prioritised the needs and preferences of end-users throughout the design process. By empathising with users and advocating for their interests, I ensured that the final product not only met regulatory requirements but also provided a superior user experience, ultimately driving user engagement and satisfaction.
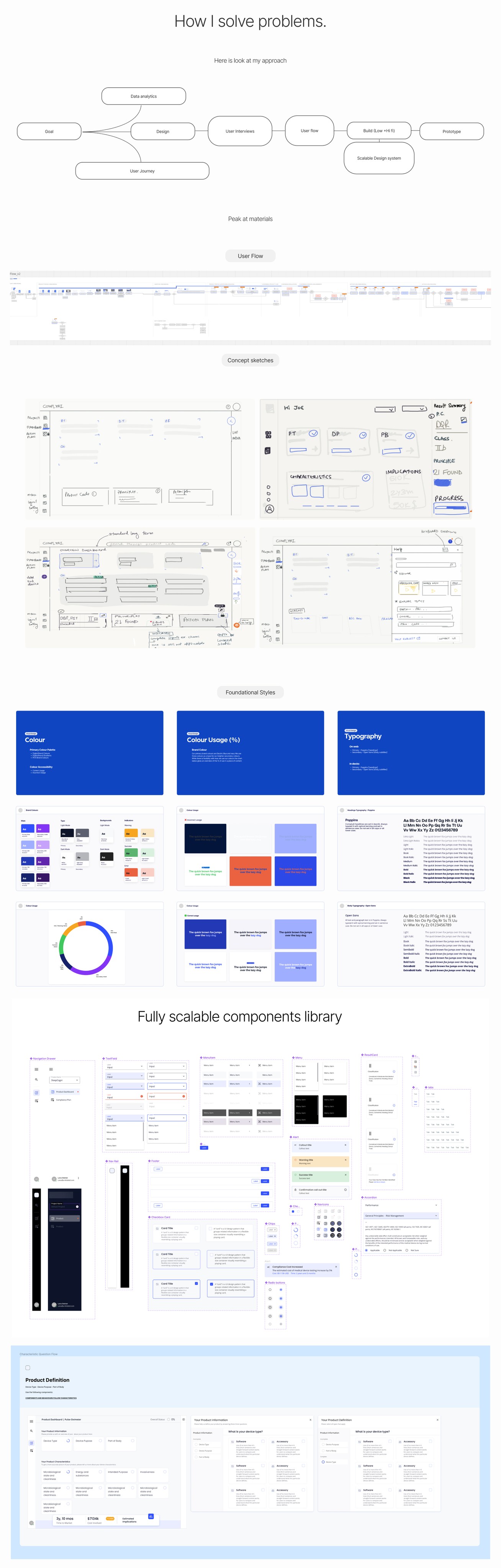
Incorporating Design System for Efficiency: Recognising the importance of efficiency and consistency in the design process, I spearheaded the implementation of a comprehensive design system. Using MUI as starting point to customise and create unified set of design principles, components, and guidelines, I streamlined the build process, enabling rapid prototyping and iteration.
Iterative Design and Usability Testing: Embracing an iterative quick design approach, I continuously refined and optimised the product's user interface based on usability testing results and user feedback. Quick prototyping and refining design elements, I ensured that the final product delivered an intuitive and seamless user experience.
Cross-Functional Collaboration: I collaborated closely with cross-functional teams, including developers, product managers, and stakeholders, to translate design concepts into tangible features and functionalities. I took initiative to have design reviews weekly basis and fostered open communication and collaboration, I ensured that design considerations were integrated seamlessly into the development process.
Handover Assets: I meticulously documented design assets to facilitate a seamless transition for the next team tasked with extending and building upon our work, ensuring consistency and coherence in future iterations.
Keypointers:
Element Digital Platform: Developed as a single source of truth, the platform offers visibility into test plans, status updates, and results, fostering collaboration among stakeholders.
Long-Term Compliance Strategy: By integrating compliance considerations early in the R&D process, we enable clients to proactively address regulatory requirements and automate re-certification as needed.
Real-Time TIC Solution: Our platform provides real-time insights and transparency throughout the product lifecycle, facilitating informed decision-making and efficient compliance management.
Expertise and Data Support: Leveraging our breadth of expertise, we assist clients in selecting materials and suppliers that best suit their R&D objectives, enhancing the efficiency and efficacy of their development efforts.
Conclusion: In conclusion, our journey to create a digital product for regulatory processes in the med tech industry has been both challenging and rewarding. By addressing the complexities of compliance head-on and leveraging digital innovation, we are poised to revolutionise how companies navigate regulatory requirements and bring products to market. With our platform, we aim to empower clients to streamline their processes, accelerate product launches, and ultimately improve patient outcomes.