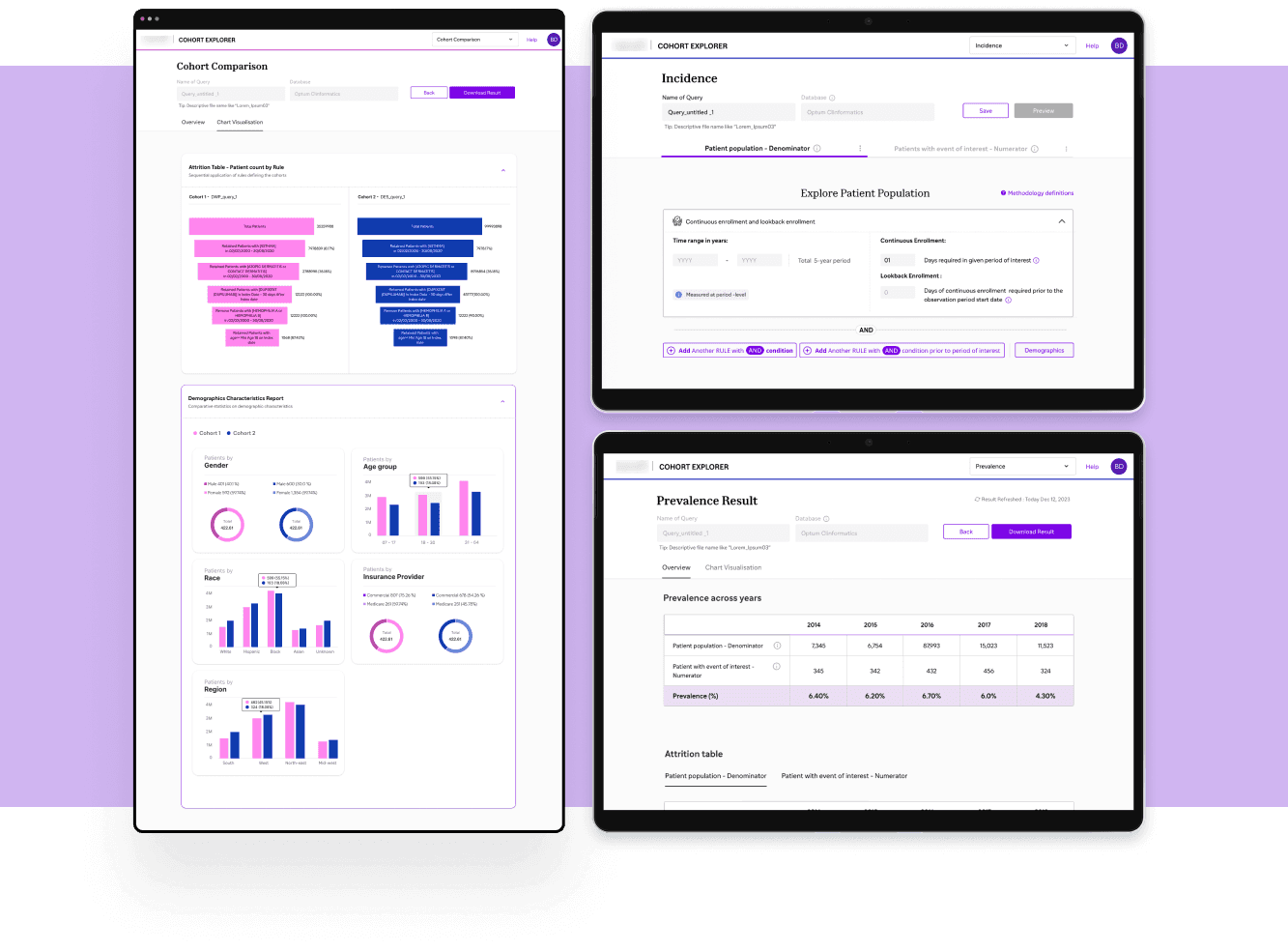
Real World Data - Cohort Counts
Case Study: Real-Time Pharma Analytics Platform Build
Introduction: In response to the evolving needs of a global pharmaceutical and healthcare company (confidential), a real-time analytics platform was developed to enable seamless data analysis and interpretation. This case study delves into the journey of building this transformative platform, addressing key challenges, methodologies employed, and the resultant impact on the organization's Real-World Data (RWD) capabilities.
Challenges Faced:
Existing platform's limited scalability and speed.
Growing demand for real-time cohort counts and analysis from a diverse user base.
Complexity of statistical methods and criteria required for accurate data interpretation.
Objectives:
Enhance existing platform with comparison, incidence, and prevalence analysis capabilities.
Ensure scalability and real-time data processing to meet the needs of 500+ users.
Simplify complex statistical methods and criteria for user-friendly interaction.
My Role and Methodology:
As the lead UI/UX Designer, my role encompassed strategic alignment with cross-functional teams, ensuring the integration of user-centric design principles throughout the development process. I facilitated weekly client presentations, providing regular readouts and incorporating user feedback to refine solutions. Additionally, I created comprehensive high-fidelity handover files in Figma, serving as a blueprint for development teams to implement the design effectively while adhering to best practices.
Research and Discovery:
Conducted expert user interviews, collaborating with a translator to understand intricate criteria and statistical methods.
Identified user pain points and requirements for real-time analytics.
Use-Case Redesign:
Leveraged existing platform functionality to redesign use-cases, focusing on user-centered design principles.
Enabled new users to effortlessly generate insights based on user-defined criteria, enhancing accessibility.
Feature Introduction:
Led the design and implementation of comparison capability with intuitive data visualisations for seamless workflow integration.
Collaborated with developers to ensure a cohesive user experience and efficient feature implementation.
Strategic Alignment and Implementation:
Championed strategic alignment across cross-functional teams, advocating for features that streamlined manual inputs and met user needs.
Ensured the adoption of user-centric design principles throughout the development process.

Key Pointers:
Facilitated faster results and scalability as operations scaled up for computation through intuitive UI/UX design.
Empowered pharmaceutical teams to compare complex datasets and manipulate data rapidly using natural language interfaces.
Interpreted real-time data as a self-serving tool, enabling users to gain insights instantaneously through user-friendly interfaces.
Provided teams with the ability to interact and converse with their data seamlessly, enhancing collaboration and decision-making.
Unlocked performance to deliver enhanced impact of RWD capabilities, driving forward pharmaceutical research and development initiatives.
Conclusion: Through strategic UI/UX design leadership, the Real-Time Pharma Analytics Platform has revolutionized the organisation's analytical capabilities. By focusing on user needs, simplifying complex processes, and enhancing accessibility, the platform has empowered users to leverage real-world data effectively, driving impactful decision-making and advancing pharmaceutical research and development.